ISO 16603 was prepared by Technical Committee ISO/TC 94, Personal safety — Protective clothing and equipment, Subcommittee SC 13, Protective clothing. It is based on ASTM F1670-98.
Introduction
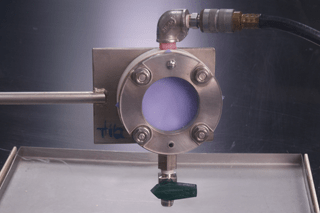
This International Standard describes a laboratory test method for measuring the penetration resistance of clothing materials to blood and body fluids. This test method uses a synthetic blood in continuous contact with the material specimen at specified set of conditions using the ISO 13994 test apparatus.
This test method is not always effective in testing protective clothing materials having thick, inner liners which readily absorb the synthetic blood.
Workers, primarily those in the health care profession, involved in treating and caring for individuals injured or sick, can be exposed to biological liquids capable of transmitting disease. These diseases, which may be caused by a variety of microorganisms, can pose significant risks to life and health. This is especially true of blood-borne viruses which cause hepatitis [hepatitis B virus (HBV) and hepatitis C virus (HCV)] and acquired immune deficiency syndrome (AIDS) [human immunodeficiency viruses (HIV)]. Since engineering controls cannot eliminate all possible exposures, attention is placed on reducing the potential of direct skin contact through the use of protective clothing.
This International Standard is concerned with protective clothing and related protective devices designed to protect against the penetration of blood or body fluids. This test method addresses only the performance of materials or certain material constructions (e.g. seams) used in protective clothing. This test method does not address the design, overall construction and components, or interfaces of garments or other factors which can affect the overall protection offered by the protective clothing.
It is emphasized that the test does not necessarily simulate conditions to which clothing materials are likely to be exposed in practice. The use of test data should therefore be restricted to broad comparative assessment of such material according to their synthetic blood penetration resistance characteristics. Testing prior to degradation by physical, chemical, and thermal stresses which could negatively impact the performance of the protective barrier, could lead to a false sense of security. Tests which assess the impact of storage conditions and shelf life on the penetration resistance for disposable products, and the effects of laundering and sterilization on the penetration resistance for reusable products, should be considered. The integrity of the protective barrier can also be compromised during use by such effects as flexing and abrasion or pre-wetting by contaminating materials such as alcohol and perspiration. If these conditions are of concern, the performance of protective clothing materials for synthetic blood penetration should be evaluated following an appropriate preconditioning technique representative of the expected conditions of use.
Medical protective clothing materials are intended to be a barrier to blood, body fluids, and other potentially infectious materials. Many factors can effect the wetting and penetration characteristics of body fluids, such as surface tension, viscosity and polarity of the fluid, as well as the structure and relative hydrophilicity or, hydrophobicity of the materials. The surface tension range for blood and body fluids (excluding saliva) is approximately 0,042 N/m to 0,060 N/m.[2] In order to help simulate the wetting characteristics of blood and body fluids, the surface tension of the synthetic blood is adjusted to approximate the lower end of this surface tension range, i.e. (0,042 ± 0,002) N/m.
Part of this method for exposing the protective clothing material specimens with synthetic blood involves pressurization of the test cell to 14,0 kPa (in Procedures A and B). This hydrostatic pressure has been documented to produce test results that correlate with a human factors validation.[3] Some studies, however, suggest that mechanical pressures exceeding 345 kPa can occur during actual use.[4] [5] Therefore, it is important to understand that this test method does not simulate all the physical stresses and pressures that are exerted on protective clothing in use. This test method can also be used as a screening test to determine which time and pressure protocol is appropriate for evaluating the viral-resistance-properties of protective apparel with a more sophisticated barrier test method as described in ISO 16604. Procedures C and D use a stepped pressurization approach with pressures up to 20,0 kPa. These procedures simulate a range of possible procedures for ranking material performance.
Given the variety of health care settings, activities, and the potential for exposure to blood or body fluids, the barrier requirements for protective clothing materials will change with the application. The choice of an appropriate test method depends on the specific application of protective clothing and its intended use. A risk assessment should be performed to determine the level of risk for determining the appropriate test method